Équipe Philippe MARIN
Neuroprotéomique et signalisation des pathologies cérébrales
Project Neuron-Glia communication during developmental and pathological neurodegeneration
PRINCIPAL INVESTIGATOR

IGF staff involved
Marie-Laure PARMENTIER
DR2 Inserm
Julien VILLENEUVE
CRCN CNRS
Charline GAL
Doctorante CNRS
Camille ENJOLRAS
IE CDD CNRS

Nervous systems are initially overpopulated with neurons and over wired. This is followed by a key period of remodeling within which a subset of inappropriate connections is removed to optimize adult functions. Neuronal elimination is also required after nervous system injury. In both cases, the clearance of resulting debris is carried out predominantly by phagocytic glia. Therefore, the selection of specific connections for elimination involves an essential communication between neurons and glia. The nervous system employs a variety of molecules to identify targets for engulfment, including chemokine signaling which is crucial for guiding glial responses. However, major gaps exist in our understanding of how chemokines mediate communication between neurons and phagocytic glia.
We recently identified a Drosophila chemokine-like protein that we named Orion. Orion plays a major role in the remodeling of the fruit fly nervous system during both development and injury, ensuring neuron-glia communication in several types of degenerating neurons. Orion is required for glial infiltration into axon bundles and for the phagocytosis of remnant axonal debris. To understand these mechanisms, our research pursues the following three objectives:
- Characterizing the topological organization of Orion upon its release
Could the way Orion is presented by neurons be the critical factor in modulating glial-receptor activation and subsequent intracellular glial responses leading either to axon bundle infiltration or phagocytosis? We focus on how neurons display Orion to glia. We aim to determine the extent to which a particular mode of presentation of Orion – Orion bound to glycosaminoglycans at the neuronal plasma membrane versus Orion “free” or extracellular vesicle (EV)-carried leads to specific glial behaviors.
- Deciphering glial signaling pathways activated by different forms of Orion
Infiltration, engulfment and phagocytosis are concomitant mechanisms that are difficult to dissociate, as glia phagocyte neurons while gradually infiltrate axon bundles.
We aim to determine the role of Orion in glial phagosome formation and maturation using Drosophila genetic and cell biology approaches. In addition, we will combine bulk and single-cell RNA sequencing in Drosophila astrocytes from different orion genetic contexts in which we can selectively block glia infiltration, phagocytosis or both and compare this to wild type. This will reveal the molecular signatures and regulatory networks specifically involved in each one of these mechanisms.
- Determining glial involvement in Tau and APP spreading during Orion-mediated phagocytosis
Alzheimer’s disease is characterized by the accumulation of intracytoplasmic aggregates of the microtubule-binding protein Tau, as well as extracellular amyloid plaques. Tau protein is thought to spread in a prion-like manner between interconnected brain regions. However, the mechanisms of Tau secretion and spreading under physiological and pathological conditions remain unclear. Our aim is to uncover the role of Orion-activated phagocytic glia in Tau / Aß42 aggregate dissemination during brain development and aging.
Overall, we use Drosophila genetic approaches (e.g., mosaic analysis with a repressible cell marker (MARCM) clone induction, binary gene expression systems …), together with biochemistry (hemolymph and EV isolation), cell biology and high-resolution microscopy techniques.
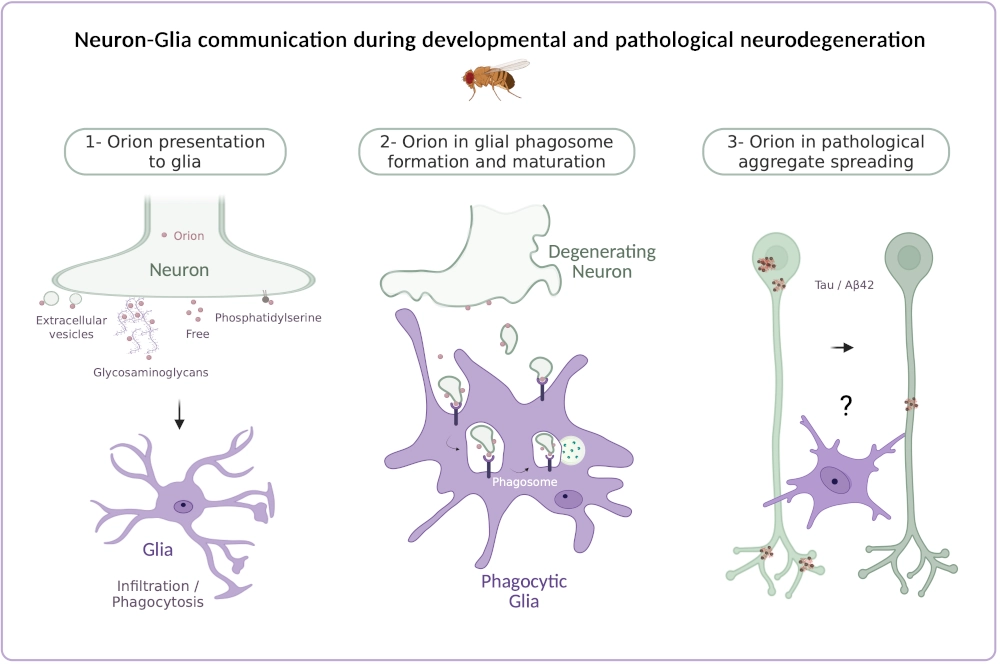
Orion, a chemokine-like signal, tags degenerating neurons for glial phagocytosis. The role of orion in Tau / Aß42 aggregate spreading will be examined during glial phagocytosis
Main publications
• Gal C., et al (2025) Cell signal, 3, 135.
• Perron C., et al. (2023) Development, 150, dev201633.
• Ji H., et al (2023) Proc Natl Acad Sci USA, 120, e2303392120.
• Boulanger A. and Dura J-M. (2022) Bioessays, 44, e2100254.
• Boulanger A., et al (2021) Nature Commun, 23, 1849.
• Boulanger A., et al (2012) PLoS ONE, 7, e40255.
• Boulanger A., et al (2011) Nature Neurosci, 14, 37.
Funding
• 2024-2028 ANR 2024-ExORION – Coordinateur
• 2022-2025 ANR 2024-ORIO – Responsable scientifique
• 2006-2007 AFM
• 2003-2005 Marie-Curie EU individual and experienced researcher fellowship
• 2002-2003 FRM
Collaborations
• Jean Maurice Dura (France)
• Hugues Lortat-Jacob (France)
• Lukas Neukomm (Switzerland)
• Chun Han (USA)
• Lee Fradkin (USA)
Alumni
• Clarisse Perron (IE, 2022-2025)
• Ouidi Fatima Ezzahara (M2, 2025)
• Charline Gal (M2, 2024)
• Salomé Ruiz-Demoulin (M1, 2021)
• Héloïse Szczkowski (M2, 2020)
• Camille Thinat (IE, 2016-2019)


