Team Chris JOPLING
Cardiac Development, Disease and Regeneration

Cardiac disease is a leading cause of death worldwide and a constantly increasing societal burden. Cardiac disease can be caused by a variety of different factors such as heart attack or hereditary genetic mutations. Our team’s goal is to leverage in vivo and in vitro models to understand the etiology of cardiac disease and how this can either be prevented or treated. Furthermore, we utilise models which are capable of cardiac regeneration in order to identify factors which could be used to induce regeneration in humans.
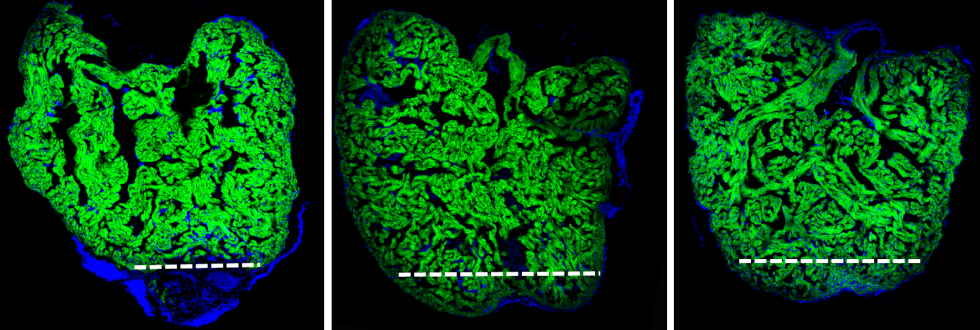
Regenerating zebrafish hearts. The first image is taken at 7 days post injury. The second image is taken at 14 days post injury. The third image is taken at 30 days post injury when regeneration is complete. The white dashed line indicates where the heart was resected.

 IGF Sud 130
IGF Sud 130 04 34 35 92 53
04 34 35 92 53-
2014HDR - University of Montpellier - France
-
2012CRCN, INSERM - France
-
2001-2006PhD, Developmental Biology, Hubrecht laboratory - Utrecht - The Netherlands
-
1999-2001MSc, Neuroscience, UCL - London - UK
-
1995-1998BSc, Biochemistry, Kings College - London - UK
-
2012-Group leader, IGF - Montpellier - France
-
2008-2011Postdoc, CRMB - Montpellier - France
-
2001-2006PhD student, The Hubrecht Laboratory - Utrecht - The Netherlands
-
2020-Scientific Director IGF Zebrafish Facility
-
2017-2020The Leducq Foundation "Research Equipment and Technology Platforms" (RETP)
-
2013-2016Marie Curie Career Integration Grant (CIG)
-
2012-2016ATIP AVENIR
- I am a molecular biologist working to understand the molecular mechanisms of cardiac regeneration

 IGF Sud 130
IGF Sud 130 04 34 35 92 53
04 34 35 92 53-
2017Habilitation to Supervise Reseach (HDR) - Univ. Montpellier - France
-
2004PhD - Cellular and Molecular Biology - Pierre et Marie Curie University - Paris VI - France
-
2000MS - Cellular and Molecular Biology - Pierre et Marie Curie University - Paris VI - France
-
1999BS - Biochemistry - Pierre et Marie Curie University - Paris VI - France
-
2017-CNRS Research Fellow - Institute of Functional Genomics - Montpellier - France
-
2012-2017Research associate - Institute of Functional Genomics - Montpellier - France
-
2007-2012Postdoctorate - Centro de Regulación Genómica - Barcelona - Spain
-
2004-2007Postdoctorate - Hubrecht Laboratory - Utrecht - The Netherlands
-
2007-2012PhD - Cochin Institute - Paris - France
-
2022-Member of the Equipment committee - Institute of Functional Genomics
-
2020-Member of the CBS2 Doctoral School board
-
2018-Member of Animal Welfare Council - Arnaud de Villeneuve campus
-
2018-2020Head of the zebrafish facility - Institute of Functional Genomics
-
2013-2017Member of the Laboratory Council (elected) - Institute of Functional Genomics
- I am interested in the role of haemodynamic forces on the normal and pathological development of the heart, mainly using the zebrafish as a model.

 IGF Sud 130
IGF Sud 130 04 34 35 92 53
04 34 35 92 53-
2009PhD - Biology - University of Montpellier - France
-
2006Master Biology & Health - University of Montpellier - France
-
2003Bachelor of Science - Biological Sciences - University of Surrey - Guildford - England
-
2019-CRCN INSERM, Jopling team, Institute of Functional Genomics - Montpellier - France
-
2016-2019CRCN INSERM, M. Pucéat Lab, UMR1251 - Marseille - France
-
2013-2016Postdoctoral fellow, M. Pucéat Lab, UMR1251 - Marseille - France
-
2010-2013Postdoctoral Fellow, S. Evans Lab - University of California San Diego - USA
-
2006-2009PhD student, Institute of Functional Genomics (Dir. J. Nargeot/B. Couette) - Univ. Montpellier II - Montpellier - France
-
2016-2019Active member of the GRRC (AVENIR group, https://sfcardio.fr/avenir), affiliated with the French Society of Cardiology (SFC)
-
2014European Society of Cardiology (ESC) Pexieder Award
-
2013Schulman Research Award - Awarded by the University of California San Diego
- My work focuses on heart development, disease and regeneration. I have a particular interest in cardiac fibrosis and how it can be regulated to promote cardiac recovery.
- Technics: molecular biology, histology, CRISPRcas9, single cell/nuclei omics, cell culture, epigenetics (ChIP….).
- Models: zebrafish, mice and iPS.

 IGF Sud 102
IGF Sud 102 04 34 35 93 26
04 34 35 93 26

 IGF Sud 130
IGF Sud 130 04 34 35 92 53
04 34 35 92 53

 IGF Sud 130
IGF Sud 130 04 34 35 92 53
04 34 35 92 53- Bachelor: Degree of Biology- Department of Biology-University of Ioannina-Greece
- Master: Ecology Master- Department of Biology-University of Ioannina-Greece
- Ph.D. : PhD: Assessment of the effects of chemical agents with environmental impact using the model organismzebrafish (Danio rerio)-University of Ioannina
- 2015-2016: Research Associate-Instute of Fisheries Research Institute-Kavala-Greece
- 2016-2022: Research Associate-University of Ioannina-Greece
- 2022-2023: Post doc Researcher-University of Ioannina-Greece
- 2023-now: Post doc Researcher- IGF Montpellier- CNRS-France
- Ecotoxicology, zebrafish, Behavioral analysis, environmental implications, pollution

 IGF Sud 130
IGF Sud 130 04 34 35 92 53
04 34 35 92 53

 IGF Sud 130
IGF Sud 130 04 34 35 92 53
04 34 35 92 53
- Rolland L, Abaroa JM, Faucherre A, Drouard A, Jopling C. The ion channel Trpc6a regulates the cardiomyocyte regenerative response to mechanical stretch. Front Cardiovasc Med. 2024 Jan 8;10:1186086. doi: 10.3389/fcvm.2023.1186086. PMID: 38259319
- Boulet F, Odelin G, Harrington A, Moore-Morris T. Nipbl Haploinsufficiency Leads to Delayed Outflow Tract Septation and Aortic Valve Thickening. Int J Mol Sci. 2023 Oct 25;24(21):15564. doi: 10.3390/ijms242115564. PMID: 37958548
- Rolland L, Torrente AG, Bourinet E, Maskini D, Drouard A, Chevalier P, Jopling C, Faucherre A. Prolonged Piezo1 Activation Induces Cardiac Arrhythmia. Int J Mol Sci. 2023 Apr 4;24(7):6720. doi: 10.3390/ijms24076720. PMID: 37047693
- Rolland L, Harrington A, Faucherre A, Abaroa JM, Gangatharan G, Gamba L, Severac D, Pratlong M, Moore-Morris T, Jopling C. The regenerative response of cardiac interstitial cells. J Mol Cell Biol. 2023 Mar 29;14(10):mjac059. doi: 10.1093/jmcb/mjac059. PMID: 36271843
- Faucherre A, Moha Ou Maati H, Nasr N, Pinard A, Theron A, Odelin G, Desvignes JP, Salgado D, Collod-Béroud G, Avierinos JF, Lebon G, Zaffran S, Jopling C. Piezo1 is required for outflow tract and aortic valve development. J Mol Cell Cardiol. 2020 Jun;143:51-62. doi: 10.1016/j.yjmcc.2020.03.013. Epub 2020 Apr 3. PMID: 32251670.
- Moore-Morris T, Cattaneo P, Guimarães-Camboa N, Bogomolovas J, Cedenilla M, Banerjee I, Ricote M, Kisseleva T, Zhang L, Gu Y, Dalton ND, Peterson KL, Chen J, Pucéat M, Evans SM. Infarct Fibroblasts Do Not Derive From Bone Marrow Lineages. Circ Res. 2018 Feb 16;122(4):583-590. doi: 10.1161/CIRCRESAHA.117.311490. Epub 2017 Dec 21. PMID
- Rambeau P, Faure E, Théron A, Avierinos JF, Jopling C, Zaffran S, Faucherre A. Reduced aggrecan expression affects cardiac outflow tract development in zebrafish and is associated with bicuspid aortic valve disease in humans. Int J Cardiol. 2017 Dec 15;249:340-343. doi: 10.1016/j.ijcard.2017.09.174. Epub 2017 Sep 24. PMID: 28986054.
- Jopling C, Suñé G, Faucherre A, Fabregat C, Izpisua Belmonte JC. Hypoxia induces myocardial regeneration in zebrafish. 2012 Dec 18;126(25):3017-27. doi: 10.1161/CIRCULATIONAHA.112.107888. Epub 2012 Nov 14. PMID: 23151342.
- Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol. 2011 Feb;12(2):79-89. doi: 10.1038/nrm3043. PMID: 21252997.
- Jopling C, Sleep E, Raya M, Martí M, Raya A, Izpisúa Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. 2010 Mar 25;464(7288):606-9. doi: 10.1038/nature08899. PMID: 20336145
Identifying the cellular and molecular mechanisms which drive cardiac regeneration
We use a combination of in vivo and in vitro models and cutting, edge techniques such as single cell RNAseq to identify the genes and processes required for cardiac regeneration.
Principal investigator
Chris JOPLING
Identifying factors involved in cardiac development and disease
Our aim is to identify factors whose dysregulation leads to congenital heart defects in humans, with a particular focus on the role of hemodynamic forces on extracellular matrix homeostasis.
Principal investigator
Adèle FAUCHERRE
Cellular interactions driving cardiac remodeling
Cardiac remodeling can be both detrimental (e.g. following myocardial infarction in mammals) or beneficial (e.g. during regeneration in zebrafish). Characterizing the processes underlying remodeling in these contexts, and notably the fibrotic response, will help identify genes and signaling pathways that can be targeted to treat heart disease.
Principal investigator
Thomas MOORE-MORRIS
Humanised in vivo models
We are developing humanized in vivo zebrafish models which will allow for high throughput testing of drug efficacy and toxicity and for assessing the impact that environmental pollutants have on development and physiology.
Principal investigator
Aurélien DROUARD




