The main neurotransmitter, glutamate, activates ion channel receptors to generate very rapid excitatory responses. More than 40 years ago, here in Montpellier, we showed that glutamate also activates modulatory receptors via G protein-coupled receptors (GPCRs), the metabotropic glutamate receptors (mGuRs). These receptors appeared very early in evolution, as glutamate was already a signaling molecule in bacteria and other single-cell species. This system, which appeared very early on, was the basis for the development of complex networks of connections, giving rise to complex analytical systems such as brains. In fact, even though mGluRs are part of the large family of GPCRs, the main targets of drugs, their activation mechanism is very different.
Unlike most GPCRs, mGluRs are constitutive dimers, formed of two identical or different subunits, and have a large extracellular domain similar to bacterial periplasmic proteins that bind amino acids, sugars, or ions. Their activation involves movement between the two subunits, leading to a conformational change in one of the membrane domains and the activation of a G protein. We showed, first for a similar receptor activated by GABA, that G protein activation results from a mechanism different from that used by other GPCRs, as the conformational change in the membrane domain is different and the G protein binds to a different site. But while most GPCRs are regulated by β-arrestins, and even signal via these proteins, nothing is known about the possible functional interactions between mGluRs and β-arrestins. We only know that the latter are not necessary for the agonist-induced internalization of mGluRs.
In the joint laboratory between the IGF “Neuroreceptors, dynamics and function” team led by Philippe Rondard, and the laboratory of Prof. Jianfeng Liu, at HUST, in Wuhan (China), in collaboration with the laboratories of Zhenhua Shao and Wei Yan, the structure of the mGlu8 receptor was solved, in five different states: inactive without ligand, inactive with an antagonist, bound to an agonist and a positive allosteric modulator with and without G protein, and with glutamate and β-arrestin.
Several surprising observations emerge from this work:
- the selective mGlu8 agonist, DCPG, fully activates the receptor, even though it does not completely close the binding domain, thereby offering multiple possibilities for developing new specific ligands,
- positive allosteric modulators do not bind in a pocket of the membrane domain, as observed with most mGluR modulators, but at the interface between the two subunits, between TM6,
- as observed with all other receptors in this family, the activated receptor is asymmetric, meaning that only one G protein can be activated,
- the receptor can also recruit β-arrestin, but in an original conformation, with the receptor being symmetric and in a state close to the inactive state, thus allowing two β-arrestins to associate with this dimer. This supports our previous findings that β-arrestin is involved in the constitutive internalization of an inactive receptor.
These new observations raise many questions about these mGluR receptors, in particular the role(s) that β-arrestins may play, which is(are) certainly very different from what is observed with other GPCRs.
This work has just been published in the journal Molecular Cell.
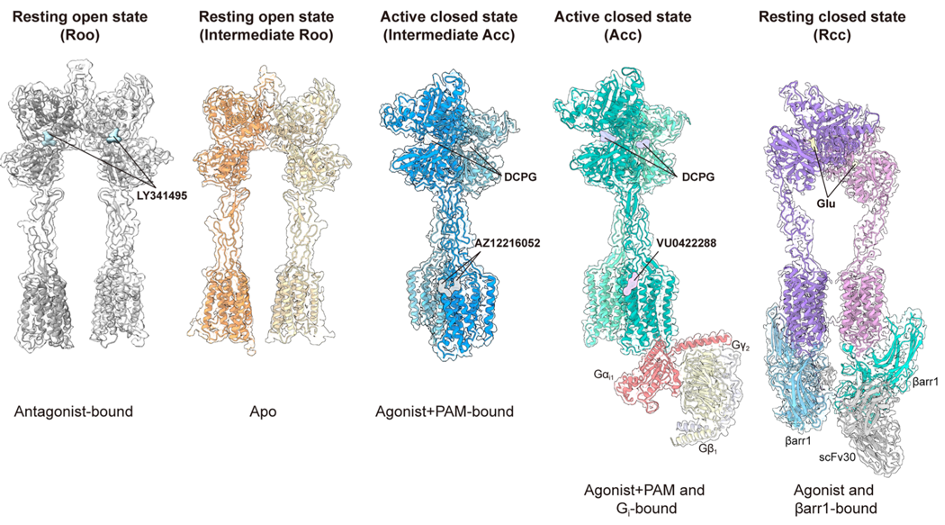
Representations of the five resolved structures of mGlu8: i) in the presence of the antagonist LY341495, ii) without ligand (Apo), iii) bound to the agonist DCPG and the positive allosteric modulator AZ12216052, iv) bound to the agonist DCPG, the positive allosteric modulator VU0422288 and the protein Gi1, and v) bound to glutamate and β-arrestin1.


