Glutamate is the main excitatory neurotransmitter in the brain, being the transmitter used by nearly 8 out of 10 synapses. Glutamate is also very toxic to neurons if present in too high concentrations, which requires sophisticated systems to properly control the sensitivity of neurons to this transmitter. For its excitatory effects, glutamate acts on very fast-acting channel receptors, but it also acts on modulator receptors coupled to intracellular transduction pathways via G proteins. The latter, called metabotropic glutamate receptors, or mGluRs, play important roles in controlling many synapses, enabling them to adapt to the glutamate present in the extracellular space.
There are 19 mGlu receptors, 8 consisting of 2 identical subunits and 11 consisting of two different subunits. Although they have been studied for over 30 years and identified as potential targets for new drugs, the mechanisms that control their presence on the surface of neurons remain poorly understood. However, understanding the dynamics of these receptors, their internalization, and their recycling is essential for estimating the effects of compounds that target these receptors in the brain.
Marta Cimadevila, under the joint supervision of Jean-Philippe Pin and Laurent Prézeau, in the “Neuroreceptors, dynamics and function” team led by Philippe Rondard, studied the internalization and recycling of virtually all mGlu receptors. Her study used innovative technology developed by Revvity/Cisbio, which allows real-time tracking of surface receptor internalization. Using this technology in control cells or cells lacking partners known to control the internalization of G protein-coupled receptors, Marta made some surprising observations. First, she observed that all mGluRs internalized constitutively and, unlike what is observed with other G protein-coupled receptors, this process requires β-arrestin. She also shows that only five mGluRs, out of the nineteen (1-1, 5-5 (and therefore probably 1-5), 3-3, and 2-3) experience a significant increase in internalization by an agonist, a mechanism that is completely independent of β-arrestins!
More work remains to be done to understand the mechanisms involved in and regulating these processes, but we can already conclude that mGluRs are very different from other GPCRs!
This work has just been published in the journal Cell Reports.
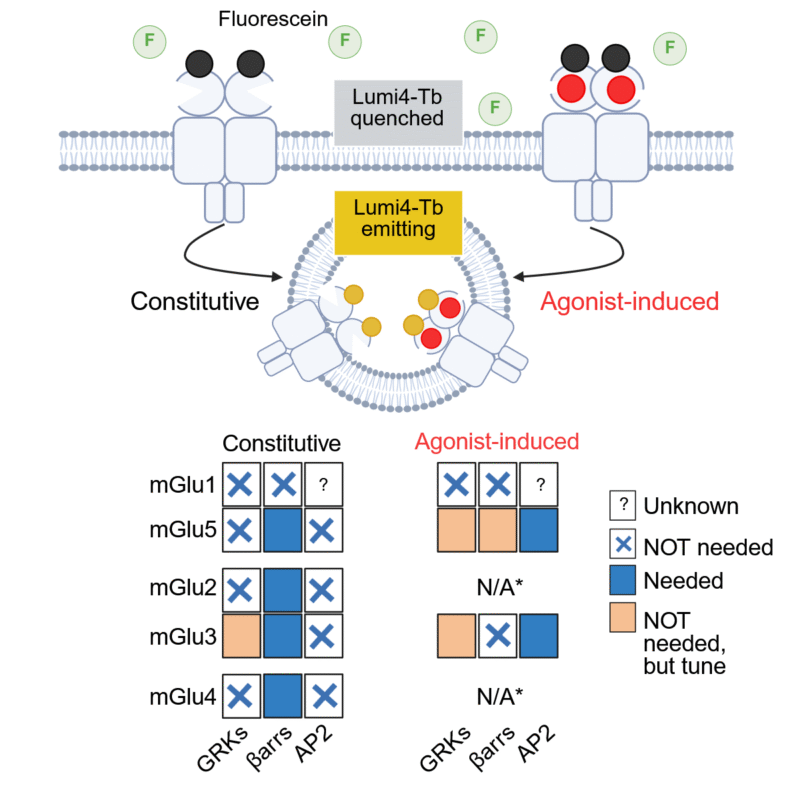
Above, diagram of the principle of receptor internalization measurement used in the study. Since the Tb fluorophore is quenched in the extracellular medium by an excess of acceptor (fluorescein), it only fluoresces when it enters the cell. Below, summary of receptors that show constitutive and agonist-induced internalization, and the role of GRKs and β-arrestins.


