STRESS, HORMONES AND PLASTICITY
Department: Neuroscience
Research subject
For additional information, please visit Team website.
A priority of the team is to clarify how hormones involved in cellular communication control key biological functions and how their dysfunction leads to pathological states. We perform longitudinal studies side by side in human patients as well as in new model non-human organisms for reaching predictability of disease trajectories and modification. This is a formidable challenge that depends on specific approaches to capture with minimal invasiveness, real time and multimodal observations notably in the practices of live in vivo microscopy, telemetric electrophysiological recordings, ethological inspection of behaviors and optogenetic or chemogenetic manipulations in freely behaving animal models. We developed such expertise over the years and move forward to explore the limits of brain networks remodeling plasticity at different organizational scales (e.g. molecules, cells, networks, organism).
The diversity of responses to stress hormones among individuals and within organs, tissues and cells is shaped by age, gender, genetics, metabolism, and the quality or quantity of exposure. However, such factors cannot explain the heterogeneity of responses in the brain within cells of the same lineage, or similar tissue environment, or in the same individual. We propose to investigate how the stress response is continuously updated by synchronized neural activity on large-scale brain networks. This occurs at the molecular, cellular and behavioral levels by crosstalk between activity-dependent and stress hormone signaling pathways, which shapes the diversity of responses based on prior experience. Such a Bayesian optimization determines (mal)adaptation to the demands of the brain, body and the external world.
The first axis of research in the lab is to understand how the diversity of glucocorticoid response throughout brain neuronal networks is essential for supporting optimal health, while its disruption may contribute to the pathophysiology of stress-related disorders, such as major depression, and resistance to therapeutic treatments. Hypothetical framework is that chronic stress accelerates the maturation/aging of neural systems in brain regions serving proximal functions while suppressing development of systems in centers supporting functions relevant later in life. Risk vs. resilience to mental disorders depends on multi-scales, long-term changes of brain development (e.g. circuits, molecules). This has tremendous implications for predicting behavioral coping styles and treatment efficacy.
The second axis of research is the ontogeny of the social brain network particularly sensitive to developmental periods and the influence of stress. Early in life, an interaction of susceptibility gene variants with stress can lead to social anxiety later in life, imposing heavy burden on society. At present, quantitative biomarkers for early diagnosis and long-term assessment of social anxiety do not exist. Without such objective biomarkers, diagnosis, monitoring and treatment adjustments rely exclusively on suboptimal clinical examination. Our goal is to develop sensitive and reliable diagnostic tests to predict the efficacy of treatments that may take months or years to manifest.
Major publications
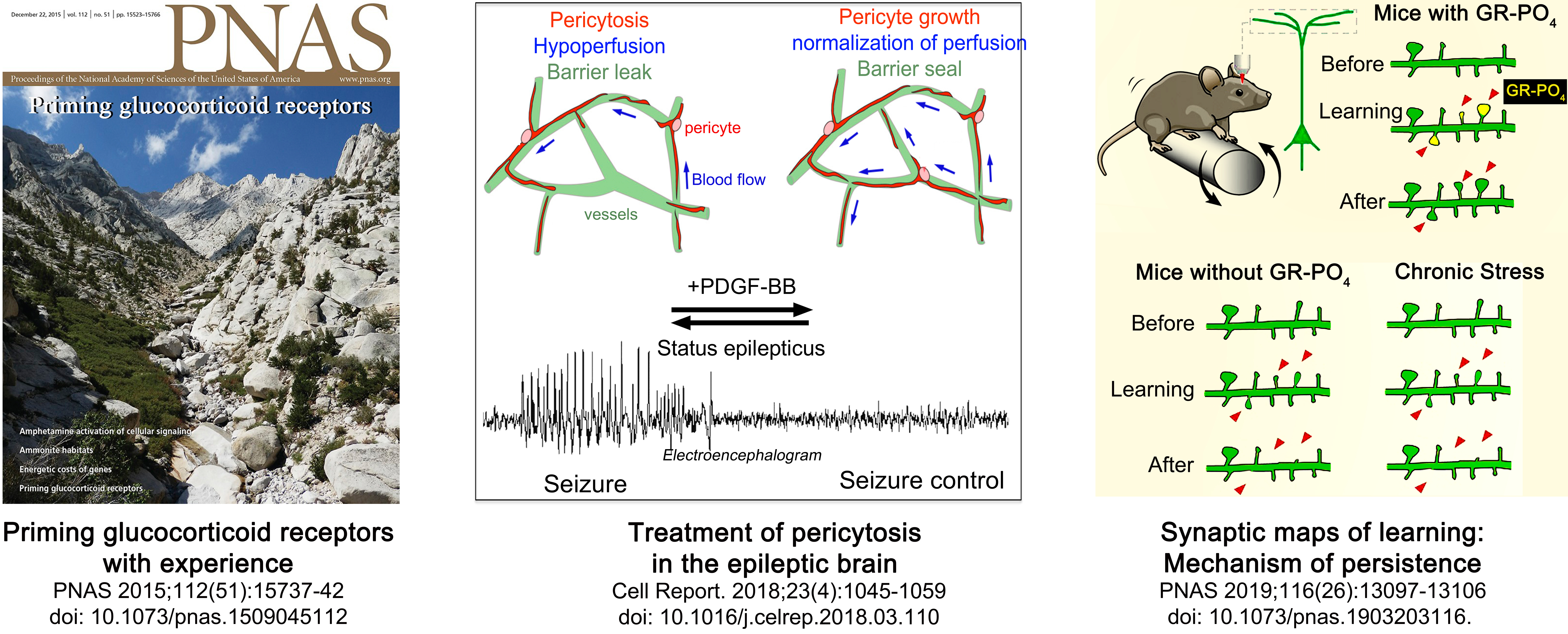
- Arango-Lievano M, Borie AM, Dromard Y, Murat M, Desarmenien MG, Garabedian MJ, Jeanneteau F. Persistence of learning-induced synapses depends on neurotrophic priming of glucocorticoid receptors. Proc Natl Acad Sci U S A. 2019 Jun 25;116(26):13097-13106. doi: 10.1073/pnas.1903203116
- Arango-Lievano M, Boussadia B, De Terdonck LDT, Gault C, Fontanaud P, Lafont C, Mollard P, Marchi N, Jeanneteau F. Topographic Reorganization of Cerebrovascular Mural Cells under Seizure Conditions. Cell Rep. 2018 Apr 24;23(4):1045-1059. doi: 10.1016/j.celrep.2018.03.110
- Jeanneteau F, Barrère C, Vos M, De Vries CJM, Rouillard C, Levesque D, Dromard Y, Moisan MP, Duric V, Franklin TC, Duman RS, Lewis DA, Ginsberg SD, Arango-Lievano M. The Stress-Induced Transcription Factor NR4A1 Adjusts Mitochondrial Function and Synapse Number in Prefrontal Cortex. J Neurosci. 2018 Feb 7;38(6):1335-1350. doi: 10.1523/JNEUROSCI.2793-17.2017
- Arango-Lievano M, Lambert WM, Bath KG, Garabedian MJ, Chao MV, Jeanneteau F. Neurotrophic-priming of glucocorticoid receptor signaling is essential for neuronal plasticity to stress and antidepressant treatment. Proc Natl Acad Sci U S A. 2015 Dec 22;112(51):15737-42. doi: 10.1073/pnas.1509045112
- Jeanneteau F, Lambert WM, Ismaili N, Bath KG, Lee FS, Garabedian MJ, Chao MV. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc Natl Acad Sci U S A. 2012 Jan 24;109(4):1305-10. doi: 10.1073/pnas.1114122109